Iso 17025 Gap Analysis Template
ISO 17025 checklist covers audit questions based on ISO/IEC requirements for each department of the testing and calibration laboratories as given below. ISO 17025 checklist is considered as very good tool for the auditors to make audit questionnaire to verify effectiveness of implemented laboratory management system. Total more than 200 internal ISO 17025 audit questions are prepared based on ISO standard.This ISO 17025 checklist shall be filled in by laboratories that are applying for, or wish to renew its accreditation. If an accredited laboratory has made big changes in the management system’s structure, the ISO 17025 checklist also shall be filled in.The paragraphs arranged in the ISO 17025 checklist are in the same order as given in NS-EN ISO/IEC 17025 (2017). In some cases they are also referred to other requirement documents. If you are thinking of preparing an ISO version Laboratory Management System (LMS) then where do you start?
Loop, a circular shopping. After use, consumers place the empty containers into their Loop totes and go online to schedule a pickup from their home. 'Together, as we bring back the milkman model of yesterday rebooted to reflect the convenience of today, we will help to eliminate the idea of waste and bring a better product experience to consumers.' With cutting-edge technology, Loop will clean the packaging so that each product may be safely reused and promptly replenished as needed at the consumer's request.To learn more about Loop, visit www.loopstore.comAbout LoopLoop is an initiative from TerraCycle, www.terracycle.com, an innovative waste management company whose mission is to Eliminate the Idea of Waste®. Operating nationally across 21 countries, TerraCycle partners with leading consumer product companies, retailers, cities, and facilities to recycle hard to recycle waste. Consumers in the pilot region of Toronto who want to sign up for Loop are encouraged to visit www.buydurable.com to leave their contact information so they can be notified when Loop officially launches and apply to become a participant.How Loop works:Once they place an order online, consumers will receive their durable products in Loop's exclusively designed shipping tote.
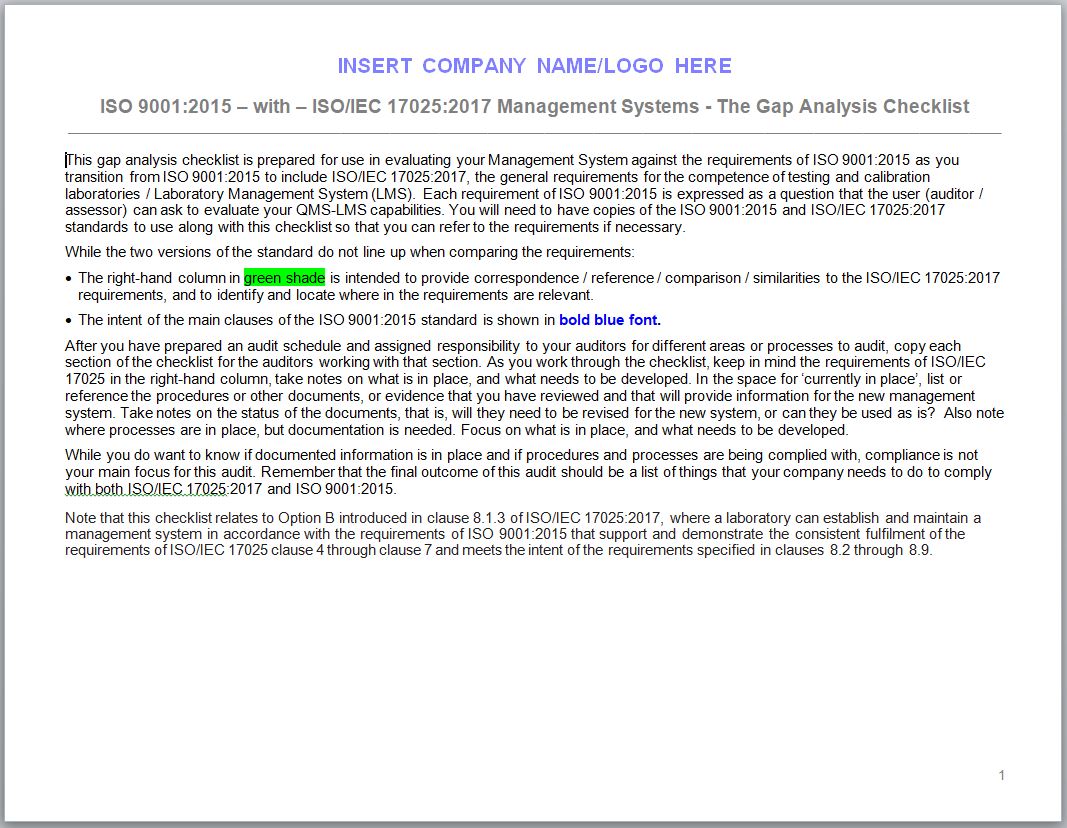
We start with a Gap Analysis, with the ISO 17025 checklist to determine the gap between your current management system and an ISO 17025 conforming LMS. A Gap Analysis is used to assess an organization's scope, readiness, and its resources for building the system. It also provides us with the data to develop a project plan for ISO implementation.This ISO 17025 checklist can be used in evaluating your Laboratory Management System (LMS) against the requirements of ISO as you transition from ISO to ISO.New requirements and / or new terminology are highlighted in bold red.The second column is intended to provide reference / comparison to the ISO requirements.Comments in the second column in bold red font indicate removed / missing requirements.
Procedures & resources for ISO 17025A copy of the ISO 17025 standard itself.A range of operating procedures and forms required by the standard.A substantial checklist questionnaire for gap analysisFrom a Project Schedular to a SOP Template.ISO 17025 distilled into a few reference sheetsAn overview for executive management, and a detailed presentation for staff.The ISO 17025 Quality KitA complete kit comprising checklists, presentations, procedures, manual, the standard itself and more.Special Offer. The ISO 17025 Quality ManualThis is an extensive template document that helps you create a compliant ISO 17025 Quality Manual. It includes the Scope and Field of Application, Normative References, Terms and Definitions, Management Requirements, and Technical Requirements.
Guidance on the completion of these templates is provided in the ISO 17025 Quality Manual Template User’s Guide, which takes you step by step through the process. See below for sample pages from both the template and the guide.The template.
Gap Analysis Template Excel Free
Nov 29, 2017 - Rather than performing a gap analysis, our laboratory is planning to be. I've noted that the format of the new version of ISO/IEC 17025 is.
Comments are closed.